Steri tek provides turnkey sterilization validation packages per iso 11137 vdmax for companies that wish to outsource their sterilization validation work.
E beam sterilization validation.
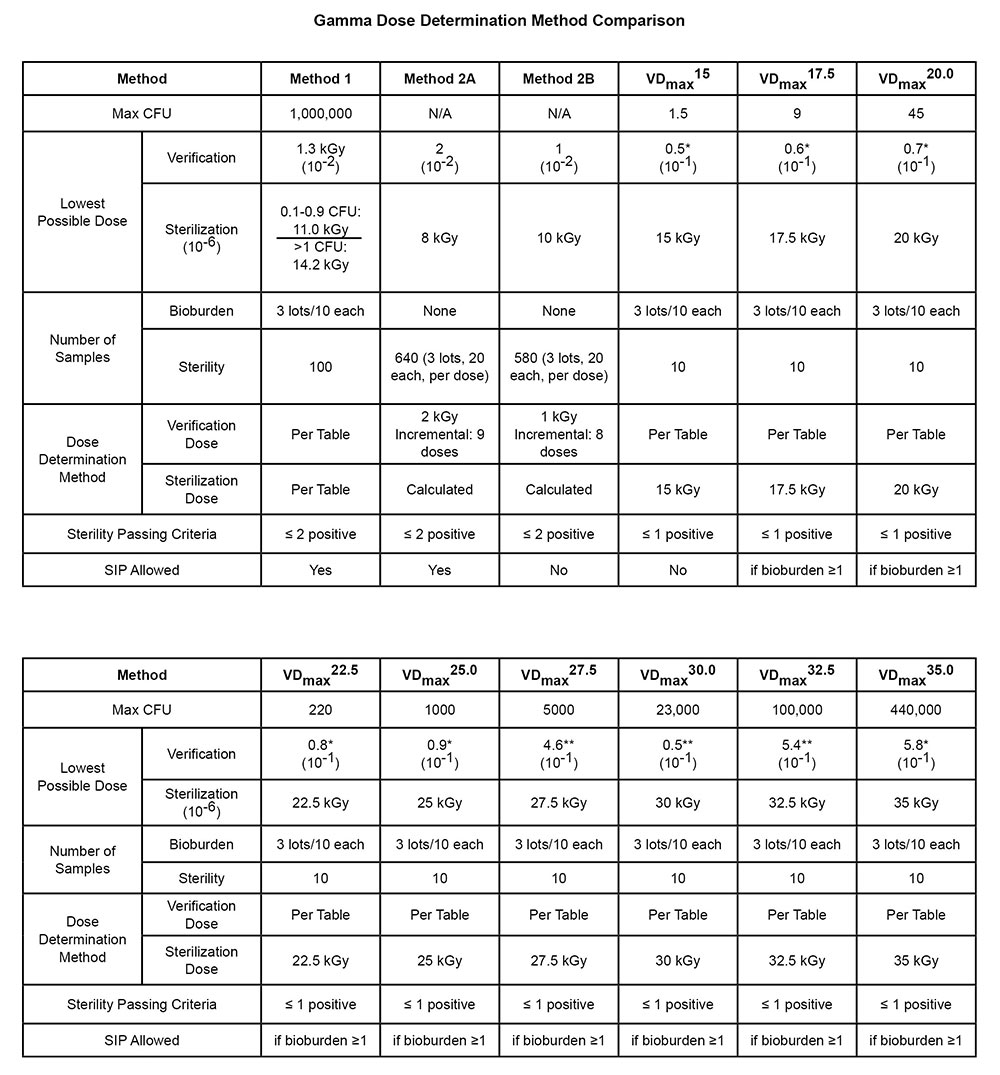
The sterilization validation requirements are set forth in the international standard iso 11137.
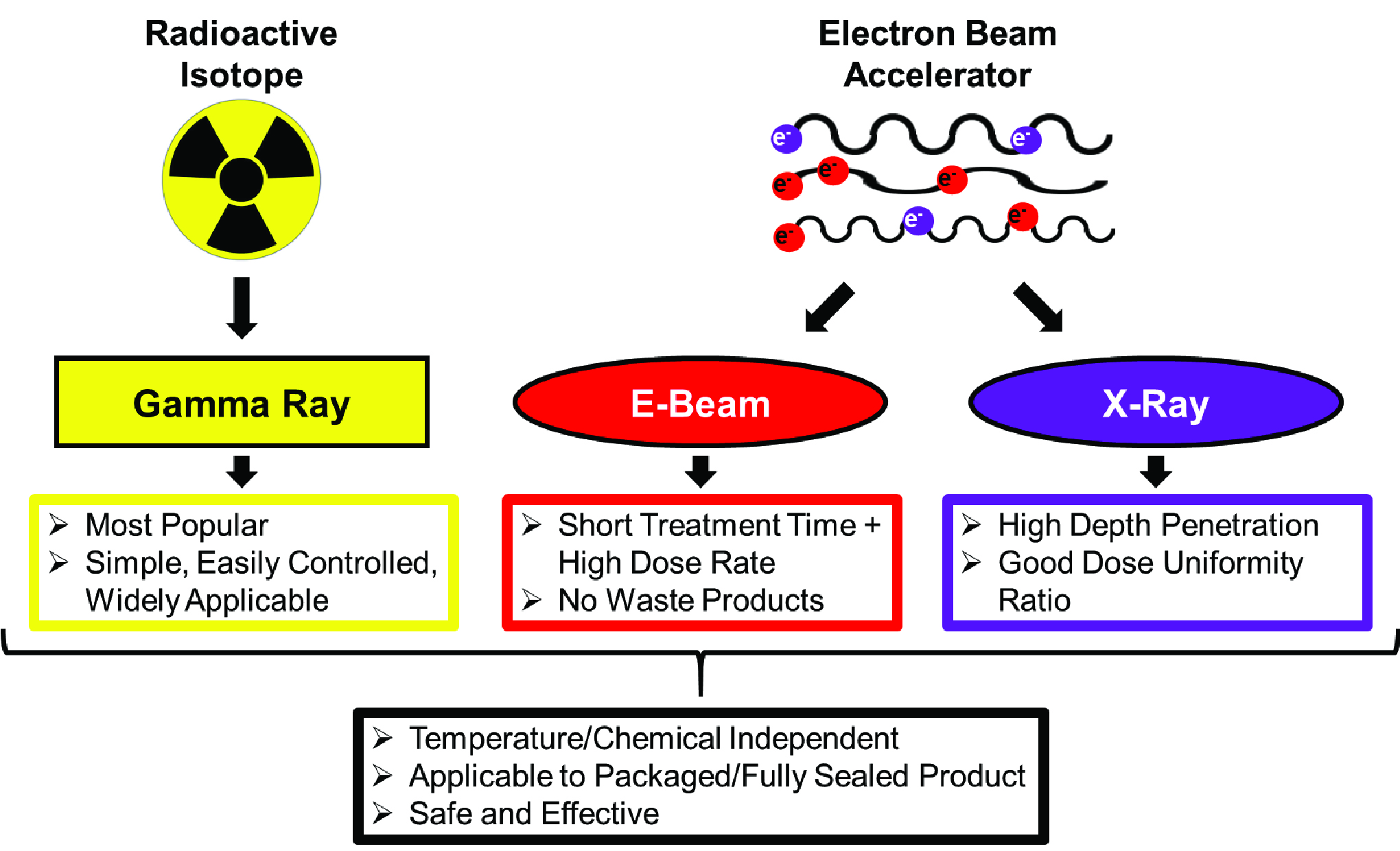
The beam a concentrated highly charged stream of electrons is generated by accelerators capable of producing continuous or pulsed beams.
To claim your product is sterile after e beam treatment you must perform several validation steps as regulated by iso 11137.
E beam services will help guide you through the ansi aami iso 11137 validation requirements for the sterilization of medical devices using radiation and usp guidelines for the sterilization of pharmaceuticals.
During his previous employment he learned the ins and outs of medical device sterilization via e beam x ray irradiation.
He now brings this knowledge to steri tek in order to help guide customers through the validation of terminal sterilization for medical devices.
Review the specific procedure s for the sterilization process selected and the methods for controlling and.
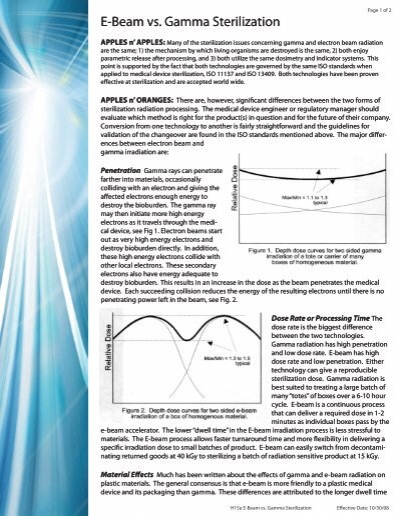
E beam sterilization is very gentle on product material and packaging when compared to gamma sterilization and is compatible with most materials.
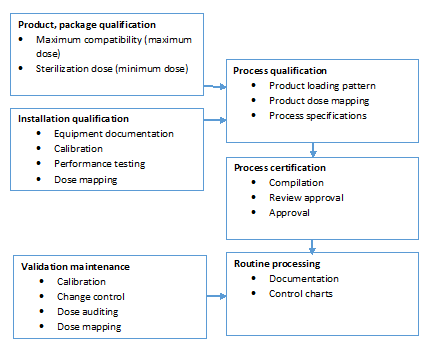
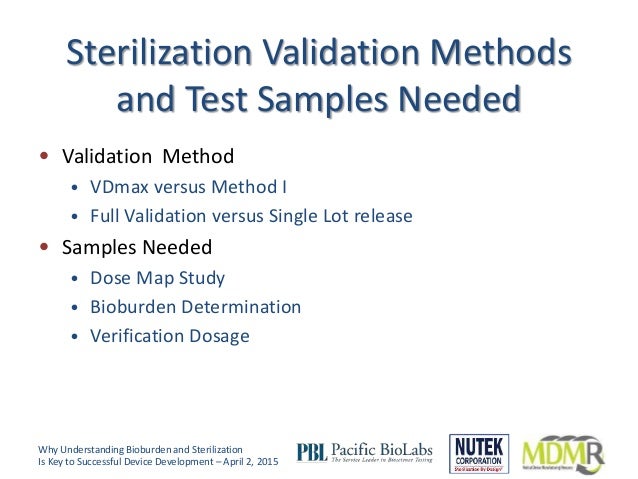
The sterilization validation process.
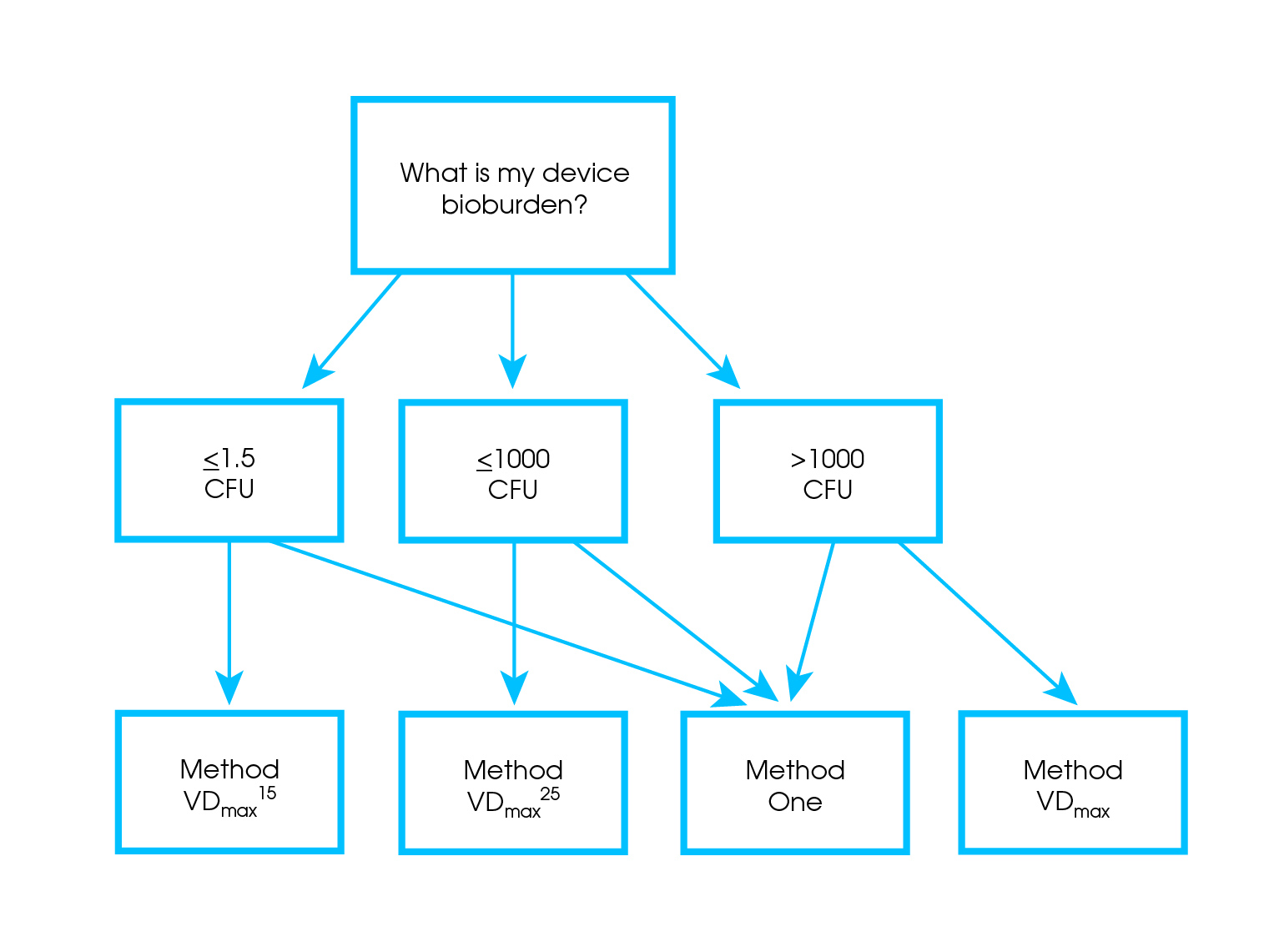
The first two steps of the process dose range validation for e beam consist of determining.
This validation process is comparable to that of gamma sterilization and it is simpler than the process for eto sterilization.
Check back in for my future blog how do i validate my medical device for e beam sterilization for details on what each step entails.
Materials testing and establishing maximum dose.
Overview of an e beam irradiation validation for healthcare products prior to beginning routine processing with electron beam e beam irradiation a product with a sterile claim needs to complete a validation process to ensure the sterility assurance level claimed is achieved.
Although e beam product penetration is highly dependent on electron energy and has reduced penetration for dense products.
Electron beam e beam irradiation is a form of ionizing energy that is characterized by its low penetration and high dosage rates.